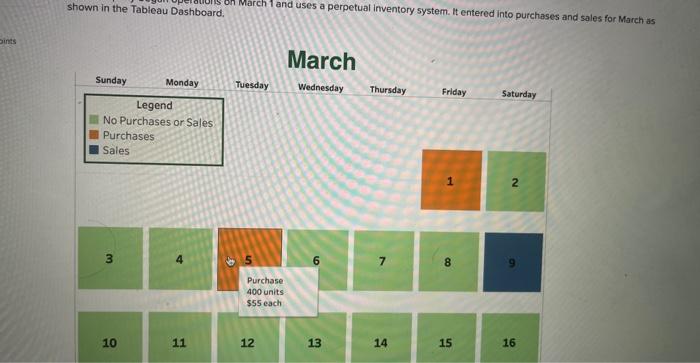
Ethylene glycol antifreeze has a density of 1.11 g cm3 – Ethylene glycol antifreeze has a density of 1.11 g cm 3, making it an essential component in automotive cooling systems and various industrial applications. Understanding its properties and applications is crucial for ensuring optimal performance and safety.
Ethylene glycol antifreeze is a colorless, odorless liquid with a sweet taste. It is composed of ethylene glycol, a diol with two hydroxyl groups, and various additives that enhance its performance and longevity. The density of ethylene glycol antifreeze is a measure of its mass per unit volume, and it plays a significant role in its ability to protect against freezing and overheating in engines.
Ethylene Glycol Antifreeze Density
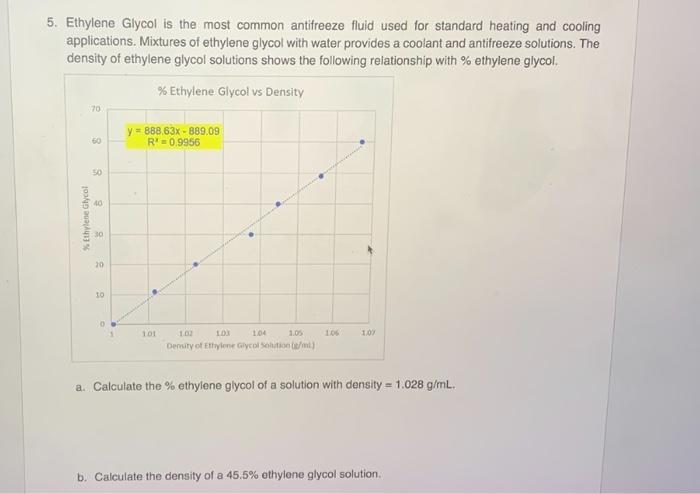
Density is a measure of the mass of a substance per unit volume. It is an important property of ethylene glycol antifreeze because it affects its ability to protect engines from freezing and overheating.
The density of ethylene glycol antifreeze is typically measured using a hydrometer. A hydrometer is a device that measures the specific gravity of a liquid. Specific gravity is the ratio of the density of a liquid to the density of water.
The density of water is 1 g/cm 3. Therefore, the density of ethylene glycol antifreeze can be calculated by multiplying its specific gravity by 1 g/cm 3.
The density of ethylene glycol antifreeze can be affected by several factors, including temperature and concentration. The density of ethylene glycol antifreeze decreases as the temperature increases. This is because the molecules of ethylene glycol expand as they are heated, making the liquid less dense.
The density of ethylene glycol antifreeze also decreases as the concentration of ethylene glycol in the solution decreases. This is because water is less dense than ethylene glycol.
Composition and Properties of Ethylene Glycol Antifreeze
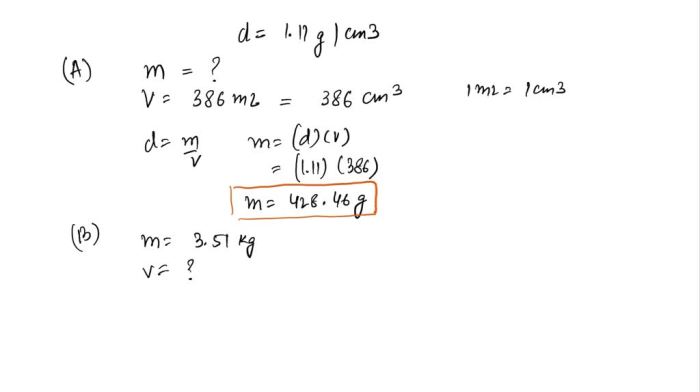
Ethylene glycol antifreeze is a mixture of ethylene glycol and water. Ethylene glycol is a colorless, odorless, and slightly viscous liquid. It is miscible with water in all proportions.
The chemical formula of ethylene glycol is C 2H 6O 2. It is a diol, meaning that it has two hydroxyl groups (-OH) attached to a carbon atom. The molecular structure of ethylene glycol is shown below:

Ethylene glycol antifreeze has several important physical and chemical properties. These properties include:
- Boiling point: 197 °C (387 °F)
- Freezing point: -13 °C (8.6 °F)
- Viscosity: 16.1 cP at 20 °C (68 °F)
- Specific gravity: 1.11 g/cm 3at 20 °C (68 °F)
Ethylene glycol antifreeze also contains a number of additives. These additives help to improve the performance and longevity of the antifreeze. Some of the most common additives include:
- Corrosion inhibitors
- Anti-foaming agents
- Lubricants
Applications and Uses of Ethylene Glycol Antifreeze: Ethylene Glycol Antifreeze Has A Density Of 1.11 G Cm3
Ethylene glycol antifreeze is primarily used in automotive cooling systems. It is also used in a variety of other industrial and commercial applications.
In automotive cooling systems, ethylene glycol antifreeze helps to protect the engine from freezing and overheating. It does this by lowering the freezing point and raising the boiling point of the coolant. Ethylene glycol antifreeze also helps to prevent corrosion and lubricate the water pump.
Other industrial and commercial uses of ethylene glycol antifreeze include:
- Deicing fluids
- Heat transfer fluids
- Brake fluids
- Cosmetics
Safety and Environmental Considerations
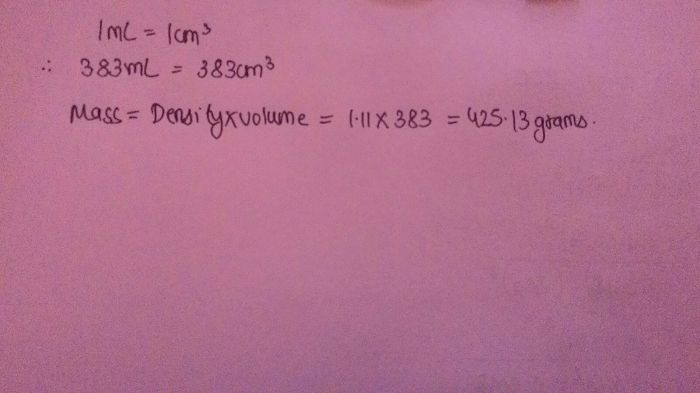
Ethylene glycol antifreeze is a toxic substance. It can be harmful if ingested, inhaled, or absorbed through the skin. Symptoms of ethylene glycol poisoning include nausea, vomiting, abdominal pain, headache, and dizziness. In severe cases, ethylene glycol poisoning can lead to kidney failure and death.
Ethylene glycol antifreeze is also harmful to the environment. It can contaminate soil and water, and it can be toxic to wildlife. It is important to dispose of ethylene glycol antifreeze properly. It should be recycled or disposed of at a hazardous waste facility.
There are a number of ways to reduce the environmental impact of ethylene glycol antifreeze. These include:
- Using extended-life antifreeze
- Recycling antifreeze
- Disposing of antifreeze properly
- Using alternative antifreeze products
Detailed FAQs
What is the density of ethylene glycol antifreeze?
Ethylene glycol antifreeze has a density of 1.11 g cm 3.
What is the chemical composition of ethylene glycol antifreeze?
Ethylene glycol antifreeze is composed of ethylene glycol, a diol with two hydroxyl groups, and various additives.
What are the primary applications of ethylene glycol antifreeze?
Ethylene glycol antifreeze is primarily used in automotive cooling systems to protect against freezing and overheating.